
Latex Binders 101: Polymer Stabilization
Chemists sometimes describe latex as a colloidal dispersion that remains stable — i.e., the particles that make up the dispersion don’t settle or cream over time. This is accomplished by a combination of ionic and steric stabilization. Keep reading..
Chemists sometimes describe latex as a colloidal dispersion that remains stable — i.e., the particles that make up the dispersion don’t settle or cream over time. This is accomplished by a combination of ionic and steric stabilization. Keep reading to understand how these two unique mechanisms work.
The word “latex” is a direct lift from Latin and means, literally, “liquid.” In the early 1800s, when it officially entered the English language, latex referred to a specific liquid — the milky white excretion found in the rubber tree and other similar plant species. The most common latex is milk — a protein dispersed in water.
Today, latex describes both the natural type and synthetic types made through a process known as polymerization. At the same time, scientists define latex more precisely as a colloidal dispersion. Most synthetic latex consists of polymeric particles, which are usually a few hundred nanometers in diameter, dispersed in water. The whole dispersion is said to be colloidally stable, meaning it can sit undisturbed without the particles settling or creaming over time.
To understand why latex behaves this way, we must unpack the term “colloidally stable.” What is a colloid? What is stabilization, and how does it contribute to the process of making the latex products we see every day, such as paints, adhesives and binders?
A Crash Course in Colloids
A colloid is a type of mixture involving two phases — a continuous phase (the dispersion medium) and a dispersed phase (the particles). The particle size of the dispersed phase typically ranges from 1 nanometer (1 billionth of a meter, or the distance from the center of an oxygen atom to a hydrogen atom in water) to 1 micrometer, with latex particles falling between 100 to 300 nanometers. There are different types of colloidal dispersions:
- a solid dispersed in a liquid, also known as a suspension or dispersion or emulsion
- a liquid dispersed in another liquid
- a gas dispersed in a liquid
- a liquid dispersed in a gas
- a solid dispersed in a gas
In any of these dispersions, the colloid particles aren’t stationary. They move in a random pattern known as Brownian motion. As they move around, they can collide with each other, and these collisions can result in agglomeration that could eventually cause coagulation — particles sticking together due to relatively weak electrical attraction occurring via van der Waals forces. As the particles coagulate, the colloid can become destabilized, which is not a desirable outcome. So how do chemists counteract the van der Waals attraction?
There are two primary approaches to preventing coagulation, and we’ll review those next.
Electrostatic Stability
The magnitude of this repulsion is dependent on two variables — the charge density on the particle surfaces and the medium surrounding them. For example, the addition of an electrolyte (such as salt) can begin to diminish the electrostatic repulsion until the van der Waals attraction can resume control and pull the colloid particles together. Eventually, this can cause the dispersion to become unstable. For negatively charged particles, this effect is particularly magnified when electrolytes with multivalent cations, such as Ca+2, Al+3 and Fe+3, are added to the medium.
Chemists rely on something called the zeta potential as an indicator of electrostatic stabilization. The magnitude of the zeta potential is directly proportional to the degree of electrostatic repulsion between adjacent, similarly charged particles in a dispersion. For molecules and particles that are small enough, a high zeta potential is a reliable marker of colloidal stability. A low zeta potential generally indicates instability and a higher risk of coagulation.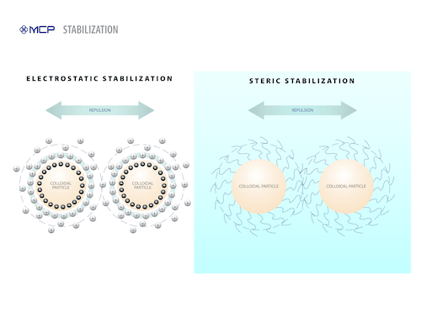
Steric Stabilization
The second way to combat coagulation involves a mechanism called steric stabilization. Steric is a term that describes how atoms are arranged in three-dimensional space, similar to the concept of spatiality. In colloidal dispersions, then, steric stabilization refers to modifying the surface of colloid particles so that they extend looping molecular structures into the continuous phase above the particle. These extensions physically prevent two particles from getting close to each other.
Chemists induce steric stabilization by adding various ingredients to the medium that “decorate” or cover the colloid particles. These ingredients include a number of chemicals, but nonionic surfactants with water-loving chains are commonly used. These additives adhere to the particles, producing an adsorbed layer with a certain thickness. When two particles get close enough, the adsorbed layers can overlap or become compressed, creating a strong repulsive force. It’s this repulsion that drives particles apart and promotes stability.
Sometimes, the stabilizing molecules remain unanchored and sit in between colloid particles. Even in this configuration, they still prevent the approach of particles and, as a result, prevent coagulation from occurring.
Ionic vs. Steric
Stability is an important concept in the production and use of latex, a delicate balancing of attractive and repulsive forces, and how chemists achieve stabilization becomes an important tool in their toolbox. Stability affects the performance of the latex; too much ionic or steric stability can prevent adhesion, filler acceptance or film formation. Too little ionic or steric stability can result in latex not remaining dispersed until time of application. The table below summarizes some of the key differences between anionic and steric stabilization:
|
Characteristic |
Ionic Stabilization |
Steric Stabilization |
|
Caused by electrostatic charges |
Yes |
No |
|
Effects of dilution |
Not affected if charge is covalently attached to the polymer |
Destabilization may occur at high latex dilutions |
|
Effects of cationic (positively charged) species |
Destabilized by cationic species |
Insensitive to cations |
|
Effects of pH |
Greater stability at higher pH |
Insensitive to latex pH unless stabilizing surfactant is pH sensitive |
The team at Mallard Creek Polymers can help you decode all of the aspects of latex manufacturing, including stabilization. We work with your team to analyze the unique needs of your application and then determine a chemical program that yields a finished product that meets those needs. Call us today to discuss your next project.
Be sure to check out the other articles in our Latex 101 series:

 Construction
Construction
 Nonwovens
Nonwovens
 Adhesives
Adhesives
 Textiles
Textiles
 Printing & Packaging
Printing & Packaging
 Paper
Paper
 Paints & Coatings
Paints & Coatings





