
It’s convenient to think of polymers as long chains, and, sometimes, that’s accurate. But polymers have a number of complex interactions — between monomers and between polymer chains — that result in recognizable architectures. These architectures..
It’s convenient to think of polymers as long chains, and, sometimes, that’s accurate. But polymers have a number of complex interactions — between monomers and between polymer chains — that result in recognizable architectures. These architectures can have tremendous impact on the properties of a polymer emulsion being developed.
Imagine for a moment that you and 19 of your friends gather outside in a large open field. Now imagine that you link your left hand in the belt loop of the next person and that she does the same and so on, until all 20 of you are linked together in a chain. Next, another group of 20 people comes out to join the first. They also link together, left hand looped through the belt loop of the adjacent person and so on, until a second chain is formed. Now try to picture those two chains of people moving next to each other. The two chains behave independently, sliding back and forth in opposite directions.
Finally, imagine that all of the free right hands of one chain grasp the free right hands of the other chain. The two chains of people are now connected, and this changes how the chains behave. They can still move side-to-side but only in a very limited way. The overall properties of the system — defined by the two interacting chains — is changed.
This example illustrates one of the most important concepts in polymer chemistry — polymer architecture. In this article, we’ll take a closer look at the various types of architectures and how modifying the basic structure of emulsion polymers changes their properties.
A Review of Polymerization
In the thought experiment described above, you and your friends represented monomers, the building blocks of a polymer chain. Of course, real-life monomers are molecules with specific chemical makeups. A few common monomers include ethylene (C2H4), styrene (C8H8), and vinyl chloride (C2H3Cl). Each of these monomers has unique properties when they exist by themselves.
Monomers aren’t generally useful in their pure form, but as building blocks for other materials, they are quite important. Polymerization is the process that connects the building blocks together into longer chains. Consider polystyrene, which is made by joining styrene molecules together to form a long carbon backbone. A long chain of polystyrene is one of the most common polymers on the planet, and it has properties that are different than styrene. The monomer styrene is a colorless oily liquid that evaporates easily and has a sweet smell at low concentrations, but polystyrene is a hard, solid plastic that is often used in products such as food packaging and laboratory ware.
Polystyrene won’t form spontaneously. The polymerization of polystyrene, known as chain or radical chain polymerization, begins when an initiator is decomposed to yield free radicals. These free radicals — highly reactive chemical species — then attack the styrene monomer, adding to one of the electrons of a double bond and releasing the remaining electron of the bond. Then, the monomer itself becomes a free radical that can react with a neighboring styrene molecule. This happens over and over again — a process called propagation — until a long chain is formed.
Just as the growth of a polymer chain can be initiated, then it can also be interrupted. There are a number of ways to stop polymerization, a process known as termination. For example, termination by combination occurs when two independently propagating chains meet at their chemically active ends; they join together and, without any free electrons available to offer a monomer, the resulting chain stops growing.
A chemist has many options available for controlling the final polymer and its properties. He can manipulate any of the following:
- The choice of the monomer
- The way in which monomers stitch together
- The length of the polymer chain
- The way two polymer chains interact
Polymer architecture depends a great deal on items two and four in the list above. That’s what we’ll address in the next section.
Polymer Structures
Scientists identify four architectures based on how polymerization occurs and how polymer chains interact with each other. Let’s consider the characteristics of each type:
- Linear — If the monomers connect through the carbon atoms, then polymerization results in a linear polymer chain. Each chain resembles a cooked
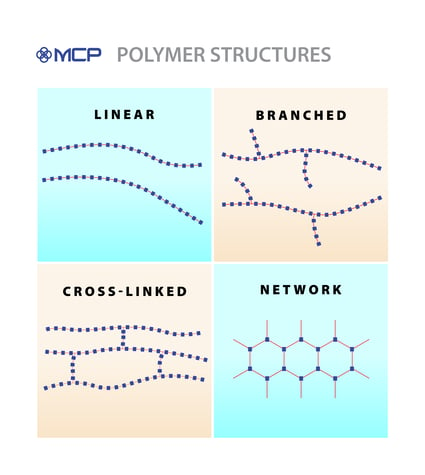 spaghetti noodle, so that two linear polymers will slip and slide past each other with only the weakest of bonds — van der Waals forces or hydrogen bonds — providing resistance.
spaghetti noodle, so that two linear polymers will slip and slide past each other with only the weakest of bonds — van der Waals forces or hydrogen bonds — providing resistance.
- Branched — Sometimes, a polymer chain can have segments branching off of the main carbon backbone. This structure is called a branched polymer chain. As you might expect, the branches prevent the polymer chains from packing closely together, though the branches themselves never fully connect one chain to another.
- Cross-linked — When the branches of a polymer chain do fully connect with a neighboring chain, the result is a cross-linked polymer. In this case, the bonds that create the ladder-like connections are covalent bonds, which are very strong. This strong bond makes most crosslinked polymers thermosetting, meaning they don’t melt, even at high temperatures.
- Networked — Finally, polymer chains can demonstrate very elaborate cross-linking until a complex network of connections is formed. This creates a networked polymer joined by many covalent bonds that is extraordinarily strong and almost completely resistant to melting.
Quantifying Cross-linking: Gel Content
Looking closer at one of the four types of polymer structures, scientists know that the density of cross-links in the polymer is an important indicator of its properties. If the density is high, the polymer will be strong because the cross-links will prevent the network from elongating, much like the human “polymer” example we described in the introduction of this article. If the density of cross-links is low, the polymer will exhibit greater elongation.
Gel content, or gel fraction, is a measurable quantification of cross-linking between polymers. To measure the gel content, polymer chemists dry and weigh the polymer. Then they wash the polymer with a solvent — acetone, toluene, or another similar liquid. In the presence of the liquid, lightly branched polymers will dissolve, but some of the polymer will swell and form a gel in direct proportion to the amount of cross-linking present. Now the chemist can filter the solvent and isolate the gel, then dry and weigh it. This weight is divided by the original weight to arrive at a gel percentage or gel fraction, according to the equation below, where W2 is the weight of the dried gel residue and W1 is the weight of the original specimen:
[W2/W1] x 100% = gel fraction (or cross-linked material)
The Journal of Polymer Science provides a simple example of what the gel fraction can reveal for a basic polymer such as poly(butyl acrylate). The properties described in this table — tack, shear and peel — are important adhesive qualities that might be required for labels, tapes or other similar products:
| Gel fraction (%) | Mw | Tack (cm) | Shear (s) | Peel (N/100 mm) |
| 1 | 340,000 | 0 ± 0 | 45 ± 12 | 11 ± 1.1 |
| 32 | 430,000 | 0 ± 0 | 1820 ± 210 | 23.4 ± 3.6 |
| 55 | 523,000 | 0.5 ± 0.2 | 40 ± 10 | 16.9 ± 1.6 |
From Journal of Polymer Science: Part A: Polymer Chemistry, Vol. 42, 1025–1041 (2004).
You can see that as the molecular weight (Mw) increases, so does the gel fraction of the polymer. And, at the same time, the adhesive properties of the polymer change dramatically. Contact us for a discussion on how to build an adhesive polymer specific to your needs.
Polymer Architects
At Mallard Creek, we’re not just chemists — we’re polymer architects. We work with your team to analyze the unique needs of your application and then determine a chemical program, taking into account key attributes such as architecture, that will yield an emulsion polymer that meets those needs.
Be sure to check out the other articles in our Latex Binders 101 series:

 Construction
Construction
 Nonwovens
Nonwovens
 Adhesives
Adhesives
 Textiles
Textiles
 Printing & Packaging
Printing & Packaging
 Paper
Paper
 Paints & Coatings
Paints & Coatings






